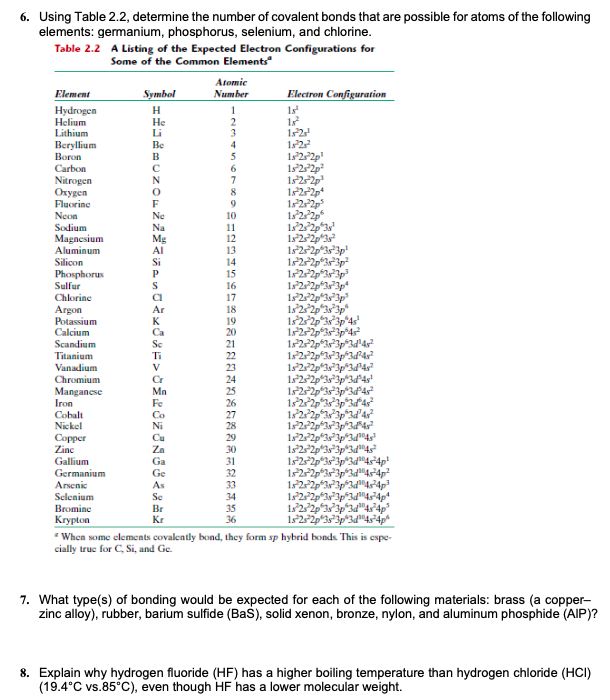
Rubber contains the isoprene 2 methyl 1 3 butadiene as its structural units which involves mostly covalent bonds.
What type of bonding would be expected for rubber.
What type s of bonding would be expected for each of the following materials.
What type s of bonding would be expected for rubber.
Hence it involves ionic bonding.
What type s of bonding would be expected for each of the following materials.
2 4fe what type s of bonding would be expected for rubber.
Hence the metallic bonding is present in the alloy.
What type s of bonding would be expected for each of the following materials.
What type s of bonding would be expected for each of the following materials.
Rubber barium sulfide solid xenon bronze nylon and aluminium phosphate.
For rubber the bonding is covalent with some van der waals bonding.
Aluminum phosphide alp all chemistry practice problems ionic and covalent bonds practice problems.
Solid xenon calcium fluoride caf2 bronze cadmium telluride cdte rubber and tungsten.
Rubber is an elastomer that occurs naturally and also make up synthetically.
Expert answer 100 13 ratings.
A ionic bonding b metallic bonding c covalent bonding with some van der waals bonding d van der waals bonding solution the correct answer is c.
Hence rubber has covalent bonding.
Rubber is composed primarily of carbon and hydrogen atoms.